Daxxify, What is Daxxify & How it Works

The U.S. Food and Drug Administration (FDA) has approved a new anti-wrinkle injection that could become a major rival to the industry leader Botox: Revance Therapeutics, Inc’s Daxxify. Daxxify, like Botox, is an injectable treatment that can treat dynamic wrinkles on the face. However, industry leaders and cosmetic treatment professionals are particularly excited about Daxxify as a competitor to Botox and other neuromuscular blocking agents because of Daxxify’s exceptionally long-lasting results. While effects of neuromodulators such as Botox, Xeomin, Dysport, and Jeuveau last for approximately 3-4 months, Daxxify has been shown in clinical trials to last in a majority of patients for up to 6 months, with some participants seeing results for up to nine months. As such, Daxxify shows a real chance of sweeping the facial injectables market, for patients who want exceptional anti-wrinkle results with only two treatments needed per year. Daxxify received U.S. approval by the food and drug administration on Thursday, September 8th, 2022 and is expected to become available for broader commercial launch in 2023.
Always on the forefront of new cosmetic treatments, experienced, board-certified dermatologist, Dr. Michele Green, is looking forward to expanding the anti-wrinkle injection options available at her New York City Upper East Side office with Daxxify. An expert in many forms of cosmetic treatment, Dr. Green is always one of the first health care professionals to adopt newly FDA approved treatments to provide patients with the leading treatments available. Dr. Green is a highly experienced injector and leader in anti-aging treatments, including injectable neuromodulators, such as Botox, Xeomin, and Dysport, dermal fillers, such as Restylane, Juvederm, and Sculptra, and a wide variety of other cosmetic and medical treatments. Well known for her expert care and high patient satisfaction, Dr. Green has been voted one of New York City’s best health care providers in such publications as Castle Connolly, New York Magazine, and Super Doctors.
What is Daxxify?
Daxxify, also known as daxibotulinumtoxina-lanm or Daxi, was created by the Nashville-based company Revance Therapeutics Inc (Nasdaq: RVNC), and is a part of a family of neuromodulators that use injections of a small amount of botulinum toxin to smooth wrinkles for a more youthful appearance. Similarly to other neuromodulators, such as Botox, Xeomin, Dysport, and Jeuveau, Daxxify is used to treat dynamic wrinkles, meaning wrinkles that develop due to repeated muscle movement. Daxxify has received FDA approval specifically to treat the glabellar lines, also known as frown lines, that appear as vertical lines on the forehead extending upward between the eyebrows. Severe frown lines can be one of the tell-tale signs of aging and Daxxify works to relax the muscles responsible for the lines to create a natural, youthful look. Daxxify differs from other neuromodulators on the market in that it is particularly long lasting. One of the reasons for the longevity of the effects of Daxxify is its peptide make up. Daxxify is the first botulinum toxin injectable treatment made up of a synthetic, proprietary Peptide Exchange Technology, meaning it does not have any human or animal products. Revance’s chief executive officer, Mark Foley, says that it was the discovery of this technology that helps to stabilize Daxxify and contributes to the unique, long-lasting results.

How does Daxxify work to treat wrinkles?
Daxxify is a neuromodulator that works to smooth dynamic wrinkles. Dynamic wrinkles form as a result of repeated muscle contraction in a given area. For example, facial expressions can result in dynamic wrinkles, including smiling, which can lead to smile lines around the mouth and crows feet on the edges of the eye, and frowning or furrowing the brow, which can lead to horizontal forehead lines or frown lines in between the eyebrows. When we are young, dynamic wrinkles only appear while we are making those facial expressions and then the lines smooth when the face is at rest. However, as we age, dynamic wrinkles become etched into the skin, forming wrinkles even when the face is at rest. These tell-tale signs of the natural aging process can be reversed with treatments available on the aesthetic market, such as Botox and now including Daxxify.
Daxxify works via the injection of the neuromodulator daxibotulinumtoxina-lanm. When injected into the muscle responsible for dynamic wrinkles, this neuromodulator works to block the nerve signal that tells the muscle to contract. As a result, the muscle is no longer engaged, smoothing out wrinkles in the area and preventing new wrinkles from forming. This anti-wrinkle temporary improvement of the Daxxify treatment can be observed for up to six months, making it the longest lasting injectable neuromodulator available on the aesthetic market.
When was Daxxify FDA approved?
Daxxify was approved by the U.S. Food and Drug Administration (FDA) on September 8th, 2022. The product was set to be approved by the FDA back in 2020 but the Covid-19 pandemic delayed the FDA representative’s visit to the Revance factory for inspection. Once the factory was properly inspected and Revance made the necessary changes to their operation, the product was finally approved in the United States. Part of receiving FDA approval for a product is to have the treatment go through rigorous clinical testing to determine its safety and effectiveness. In clinical trials, Daxxify was shown to be 98% effective after one month of treatment, with 80% of patients still noticing effects after 4 months, and roughly half experiencing improvements still after 6 months. Some patients were still seeing the results of the treatment after nine months. In clinical trials, no serious side effects were observed as a result of the treatment.
Currently, Daxxify is only FDA approved to treat the frown lines, or vertical lines that appear between the eyebrows when you frown or furrow your brow. However, Revance is seeking approval for Daxxify treatment on other facial wrinkles, such as forehead lines and crow’s feet. Furthermore, Revance believes that, like Botox injections, Daxxify can be used to treat medical conditions, such as neck spasms, excessive sweating, and cervical dystonia.
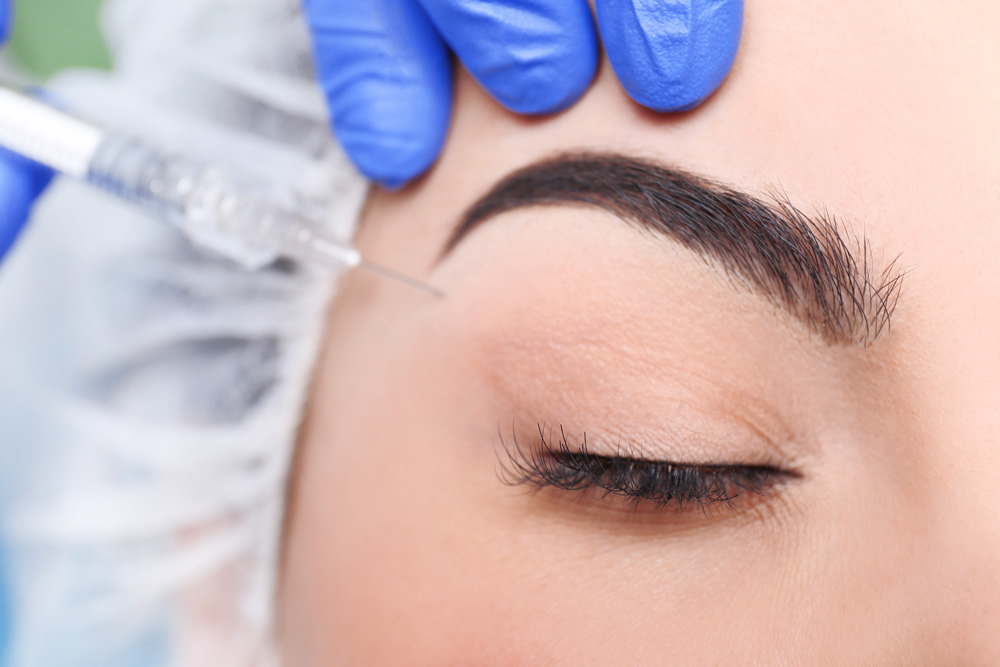
Daxxify vs Botox: What are the similarities and differences between Botox and Daxxify?
There are many similarities between Botox injections and Daxxify injections. Both are neuromodulators that work in much the same way by blocking the nerve signals from the brain to the muscle, resulting in the cessation of muscle contractions to smooth out dynamic wrinkles. Both Botox and Daxxify are FDA approved to treat glabellar lines, however, they differ in that Botox is also approved to treat other cosmetic concerns, such as forehead lines and crow’s feet, and medical concerns, such as upper limb spasticity, cervical dystonia, excessive sweating, and overactive bladder. Daxxify is expected to be considered for off-label treatment of these conditions – meaning it will work to treat this conditions but has not been FDA approved to treat them. While Botox and Daxxify work in similar ways to smooth out wrinkles, they differ in their composition in that Daxxify is the only neuromodulator available on the market that does not use human or animal products as part of the composition. Botox is made from what is called a human serum albumin whereas Daxxify uses a proprietary Peptide Exchange Technology. Revance representatives believe that this contributes to Daxxify’s longevity. Botox and Daxxify also differ in how long the effects of the treatment last, with Botox results lasting for 3-4 months and Daxxify results lasting for up to 6 months.
Does Daxxify have any side effects?
Clinical trials demonstrated that Daxxify does not have a significant side effects or safety concerns. As with any injectable treatment, patients may experience redness, swelling, bruising, or mild discomfort at the injection site for up to seven days after treatment. These mild side effects will resolve themselves on their own. Dr. Green recommends that patients avoid taking blood thinners before treatment in order to minimize the chance of bruising at the injection site. Clinical trials did reveal some uncommon side effects of Daxxify treatment include headache (6% of patients) and eyelid ptosis, or drooping eyelid (2%) of patients.
Why is Daxxify trending?
September 8th, 2022, the day of Daxxify’s FDA approval, saw many important headlines, including the passing of Queen Elizabeth II and the succession of King Charles. On that day however, Daxxify was trending as well due to FDA approval of the innovative new treatment. Daxxify is poised to become an important player in the facial injectables market and could become a major competitor for the market leader, Botox. FDA approval for the treatment has been in the works for years and with the official approval released, patients can expect the Daxxify treatment to become available sometime in 2023.

Is Daxxify a Botox rival or Botox competitor?
Currently, the most widely used neuromodulator on the market is Botox treatment, which accounts for 70% of the market share for botulinum toxin injectable treatments. Botox was first sold by the company Allergan before being bought by AbbVie and has continued to be a leader in anti-wrinkle treatment even among its already existing competition, which includes Xeomin by Merz Pharmaceuticals, Dysport manufactured by Ipsen, and Jeuveau by Evolus. Experts in the field believe that Daxxify could become a major competitor for Botox, due to the fact that the effects of the Daxxify treatment are much longer lasting than Botox – almost double. Where Botox lasts for 3-4 months, Daxxify can work for up to 6 months, meaning patients would only need to get two touch ups per year as opposed to three or four. Until the broader commercial launch, it remains to be seen how fierce the competition between Botox and Daxxify will actually be. While Daxxify will be needed less frequently for touch ups, it may not be less expensive per year than Botox treatment as Daxxify is set to be branded as a luxury treatment, though exact cost information is yet to be revealed.
How to get started with Daxxify
Now that Daxxify treatment as been FDA approved as an anti-wrinkle treatment for frown lines, it will soon become widely available for public use. Expert, board-certified dermatologist Dr. Michele Green in NYC is always adding newly approved treatments to her practice and is set to be one of the first offices to provide Daxxify to her patients. If you are looking for a long-lasting, anti-aging solution that smooths away wrinkles, Daxxify may be the best treatment option for you. To learn more about Daxxify and other cosmetic non-invasive procedures call our New York City office at 212 535-3088 or contact us online to book an appointment today.
 212-535-3088
212-535-3088