Candesant Biomedical & Brella SweatControl Patch
Primary axillary hyperhidrosis, a condition known colloquially as excessive underarm sweating, is characterized by overactive sweat glands and sweat production unrelated to high activity, heat, or high-stress situations. Sweating is a normal function of the body, helping to secrete toxins and regulating temperature to keep the body cool—however, patients who suffer from hyperhidrosis experience sweat production beyond these standard functions. Excessive underarm sweating can create feelings of embarrassment and shame, ruin clothing, and keep patients from the highest quality of life. Luckily, there are various treatment options available at Dr. Michele Green’s office in NYC that can help reduce excessive sweating and improve the quality of life for those who struggle with it.
A reported 15.3 million Americans, approximately 4.8% of the population, have been diagnosed with hyperhidrosis; of those, two-thirds experience excessive sweating in the underarm region. However, due to the embarrassment associated with the condition, the International Hyperhidrosis Society believes that these numbers underestimate the actual number of Americans with hyperhidrosis and that one in three Americans experience excessive sweating. The current treatments for hyperhidrosis, including Botox injections, are available at Dr. Michele Green’s private dermatology office in New York City. But soon to become available to healthcare providers nationwide is a new solution created by Candesant Biomedical: the Brella SweatControl Patch.
Announced in mid-March of 2023, the Brella 3-minute SweatControl patch is a revolutionary axillary hyperhidrosis treatment option. The newly FDA-cleared medical device is a single-use disposable patch that can be placed on the patient’s underarm region to suppress sweat production in the treatment area. Using Candesant Biomedical’s targeted alkali thermolysis, this non-invasive treatment causes controlled, targeted destruction of the sweat glands for long-lasting relief from excessive sweating. Results from the SAHARA pilot study of the effectiveness of the treatment were revealed at the 2023 annual meeting of the American Academy of Dermatology, demonstrating that the Candesant Brella sodium sheet is a safe and effective treatment option for primary axillary hyperhidrosis that requires no downtime and no significant adverse events. With the FDA clearance of Brella, the innovative treatment will become available to select U.S. markets nationwide on January 1st, 2024.
One healthcare provider who is always up-to-date on the newest and trending treatment options is expert, board-certified dermatologist Dr. Michele Green. Dr. Green has treated patients in her Upper East Side New York City dermatology office for over 25 years. Highly experienced in treating various cosmetic and medical concerns, Dr. Green offers procedures such as Botox, dermal fillers, laser resurfacing, chemical peels, microneedling, and much more. Well-known for high patient satisfaction, Dr. Green has been frequently voted one of the best healthcare providers in New York City by such publications as Castle Connolly, Super Doctors, and New York Magazine.

What is the new FDA-approved sweat patch?
FDA-approved in April of 2023, the Brella SweatControl Patch created by Candesant Biomedical is the newest innovation in addressing severe underarm sweating, known medically as primary axillary hyperhidrosis. The patch works via the scientific principle that when alkali metal mixes with water, it causes a heat reaction, which creates controlled destruction of the sweat glands when applied to the armpit. The treatment is unique because it is a non-invasive, needle-free, highly effective sweat-reduction option for patients who fall into a 3 or 4 on the Hyperhidrosis Disease Severity Scale (HDSS). After clinical trials with endpoints of determining if there are any severe adverse events and if the treatment reduces the HDSS to a 1 or 2, the Brella SweatControl Patch was proven safe and effective at reducing sweat by 50% for four months or more. The treatment will become widely available to healthcare providers on January 1, 2024. However, Candesant Biomedical also offers a Brella Early Experience Program to select nationwide healthcare providers focusing primarily on hyperhidrosis treatment. Dr. Michele Green, always on the cutting edge of new therapies, will offer Brella SweatControl treatment in the office as soon as possible.
What is axillary hyperhidrosis (excessive underarm sweating)?
Primary axillary hyperhidrosis is a medical condition characterized by excessive sweating in the armpit region that occurs outside of intense activity, heat, or stress. It is normal and essential for sweat to be produced during exercise, heat, or moments of anxiety, as sweat glands produce water and minerals to expel toxins and regulate body temperature. However, hyperhidrosis occurs when patients experience excessive sweating outside these circumstances, leading to embarrassment, feelings of self-consciousness, ruined clothing, and decreased quality of life. Hyperhidrosis can also be associated with other symptoms, such as body odor, skin irritation, and an increased risk of skin infection. While 15.3 million Americans have been diagnosed with this condition, it is estimated that nearly 85.2 million experience excessive underarm sweating but are uncomfortable discussing their situation with their healthcare provider. According to results published by the American Society for Dermatologic Surgery, 58% of patients seeking cosmetic procedures are most bothered by their axillary hyperhidrosis. With the U.S. Food and Drug Administration (FDA) clearing of Brella, excessive sweating treatment can become a quick and straightforward option for patients.
What is the Brella 3-minute SweatControl Patch?
With the FDA approval of the Brella SweatControl patch, there is a new, innovative solution for excessive underarm sweating. Brella is a single-use disposable patch that can be placed on the underarm region, and after only three minutes, the sweat production in the treatment area will be reduced for up to four months. The Brella sodium sheet reacts with the water in the sweat produced under the arm, producing thermal energy that carefully inactivates the sweat glands. Candesant’s Brella uses the company’s patented targeted alkali thermolysis technology – or TAT technology – to significant effect and, in a report given at the annual meeting for the American Academy of Dermatology and published in PRNewswire, the treatment was shown to produce statistically significant results in a double-blind study with no severe adverse events. This quick, safe, and effective treatment could change how excessive underarm sweating is treated, making a solution more accessible to patients.
What is Candesant Biomedical?
Candesant Biomedical is a MedTech innovator company based out of San Francisco dedicated to producing new medical devices to safely and effectively treat hyperhidrosis. Niquette Hunt, founder and CEO of Candesant Biomedical, runs the company with eight board members, providing non-invasive treatment options and innovative technology for the medical and dermatological fields. Their newly FDA-cleared Brella SweatControl patch will be available to select medical providers in late summer via the Brella Early Experience Program and to all healthcare providers on January 1, 2024.

What is Brella used for?
Candesant’s Brella SweatControl Patch treats excessive sweating in the underarm region of the body. Excessive underarm sweating, known medically as primary axillary hyperhidrosis, is a condition in which overactive sweat glands produce sweat often and uncontrollably, making it very difficult to treat. Brella is the first medical device to employ Candesant Biomedical’s patented alkali thermolysis technology to disable the sweat glands in the patient’s underarms to reduce excessive sweating for up to four months. Proven to be highly effective in clinical trials, Brella SweatControl patches reduce underarm sweating by up to 50% with optimal results. According to the Brella website, the treatment is ideal for patients who are frustrated, annoyed, or embarrassed about excessive sweating that occurs even when not in a warm environment or exercising.
How does Brella work?
The Brella SweatControl patch is made of an alkali metal with an adhesive backing that sticks to the patient’s underarm where excessive sweating occurs. The sodium sheet works via the patented process called targeted alkali thermolysis, which is the principle that when the alkali metal mixes with the water in the sweat, it will result in a thermal energy reaction. The adhesive overlay is placed on the treatment area for three minutes, and in that time, the sodium on the sheet mixes with the sweat, which is 99% water, and produces a small amount of heat energy. This heat energy localized to the treatment site causes controlled destruction to the sweat glands in the area, which causes the glands to create less sweat, reducing sweating by up to 50%. The effects of the treatment last for three to four months and can be repeated when they begin to wear off.
What is the Brella sweat treatment process?
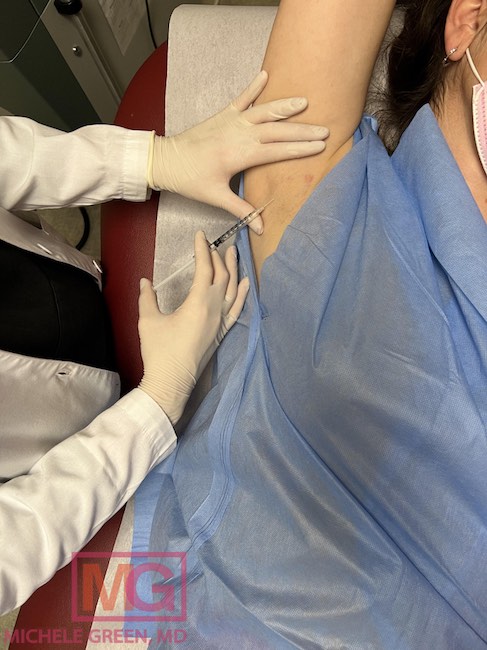
Candesant’s Brella is a quick and straightforward procedure that produces excellent effects up to four months post-treatment. The treatment must be performed by a trained healthcare provider, such as Dr. Green, and will take place in her office. One of the significant advantages of Brella Sweat Control treatment is that it is simple to perform, non-invasive, and needle-free, and there is almost no risk of developing side effects. Before beginning, Dr. Green will check the armpit area to ensure no active abrasions or irritation on the skin. To apply the adhesive, healthcare providers will begin by properly cleaning and drying the treatment area under the arms. Then, the Brella patch is placed on the skin and left for three minutes, after which your healthcare provider carefully removes the patch. While the patch is in place, patients may feel heat in the treatment area, which is an expected part of the treatment process and should not cause discomfort. The site is cleansed, then the procedure is complete and requires no downtime afterward, meaning patients can immediately return to their regularly scheduled activities. Patients should begin to experience the results within one week of receiving the treatment.
Is the procedure permanent?
Brella is a long-lasting but temporary procedure to reduce excessive underarm sweat. It takes approximately one week following the treatment for the results to take effect. Still, after one week, patients can experience up to a 50% reduction in sweat production for up to four months, according to a study published in the Journal of the American Society for Dermatologic Surgery. When the effects of the treatment begin to fade, patients can return to their healthcare provider to have the procedure repeated to maintain the results.

How do I permanently stop hyperhidrosis?
While Brella is a long-lasting treatment option for hyperhidrosis, the treatment results are not permanent. That said, Brella SweatControl treatment can be repeated every 4-6 months – when the effects begin to fade – to maintain the treatment results. Patients looking for a permanent solution to primary axillary hyperhidrosis may need to turn to a surgical procedure, such as excision, liposuction with curettage, and a sympathectomy. While these treatments are permanent, the pricing is much higher, as is the risk of serious adverse events. Surgical procedures may result in permanent scarring, inability to raise the arms fully, bleeding, hair loss, and compensatory hyperhidrosis, when excessive sweating occurs in other locations to compensate for the inability to sweat in the armpits. For that reason, Dr. Green recommends a treatment like Brella or Botox injections, which are still highly effective at reducing the effects of hyperhidrosis without the risk of severe side effects.
What are the side effects of the Brella?
According to the double-blind SAHARA study in the American Society for Dermatologic Surgery journal, no significant adverse events or side effects were associated with the Brella treatment. Some patients experienced mild to moderate side effects, including redness and irritation at the treatment site, that quickly resolved after the procedure. Some patients may experience tenderness or increased sensitivity in the armpits for up to 48 hours after treatment. Still, these symptoms will also go away without needing any medical intervention. The procedure has no downtime or recovery time, meaning patients can immediately return to their regularly scheduled activities.
How effective is the sweat patch?
Before the U.S. Food and Drug Administration can clear any medical device, sufficient studies must be done to determine via clinical trials that the device or treatment is safe for patients and effective for its intended use. Brella was FDA-cleared after findings from a double-blind, sham-protected SAHARA study in which 110 patients with severe underarm sweating participated, receiving either the Brella or a sham treatment. The participants were surveyed for 12 weeks to determine the effectiveness and safety of the treatment for severe axillary hyperhidrosis. When the findings were presented, the research showed that the Brella SweatControl patch significantly affected sweat production, with 64% of patients who received the Brella treatment seeing a reduction in sweat production. 60% of the patients who received the Brella sodium sheet saw a 50% reduction in the amount of sweat produced compared to only 33% of those who received the placebo. When asked about how their quality of life had changed after treatment, patients who received the Brella patch reported a significant improvement.
Do underarm sweat pads work?
As part of the FDA approval process, the Brella SweatControl Patches underwent rigorous clinical trials to determine whether severe side effects were associated with treatment and whether sweat production was significantly reduced to HDSS level 1 or 2. In the SAHARA study of 110 patients, half received Brella patches while the others received a placebo. The study showed that 64% of participants who received Brella SweatControl treatment experienced a 50% reduction in underarm sweat production compared to 33% of the group that received the placebo (control group). Patients chosen for the study had an HDSS level of 3 or 4, and those receiving Brella treatment saw reduced HDSS levels of 1 or 2, demonstrating the effectiveness of the treatment. The clinical trials for Brella also showed no severe side effects associated with treatment. Instead, side effects were mild, including irritation and redness for up to 48 hours post-treatment.
Are sweat pads worth it?
Brella SweatControl Patches offer a new way to treat excessive underarm sweating without irritating topical products, oral medications with serious side effects, injections, or surgery. While the pricing for treatment has not yet been released, Brella patches are predicted to be a less costly alternative to other treatment options. The procedure is quick – only three minutes per armpit – and involves minimal discomfort – only some heat at the treatment site – with long-lasting results. There is no risk of severe side effects, and no downtime is required post-treatment, meaning patients can immediately return to their regular activities. Brella Sweat Control Patches will be very popular when they become available on January 1, 2024.
What is the best sweat blocker?
Several hyperhidrosis treatment options are available, but not all are satisfactory to patients. Research presented by Candesant Biomedical shows that as many as 80% of people attempting to treat hyperhidrosis are looking for different treatment options. 63% of those surveyed say they are unhappy with prescription topical antiperspirants, 45% are dissatisfied with oral treatments, and 55% are unhappy with other topical treatments, such as wipes. Brella SweatControl patches offer an alternative to these treatments that is highly effective at reducing sweat production by up to 50%. Topical treatments often cause skin irritation throughout their use, whereas patients treated with Brella may have skin irritation for only 48 hours post-treatment. Oral treatments for hyperhidrosis are often associated with significant side effects, including dizziness, blurred vision, and drowsiness, whereas Brella patches have no serious adverse events.
Another effective sweat blocker is Botox injectable treatment that blocks neurotransmitters at the injection site to prevent sweat secretion by the sweat glands. Botox treatment has been FDA-approved to treat hyperhidrosis in the armpits, forehead, and hands. Botox treatment is also safe and effective, with no downtime following treatment and effects lasting up to three to four months. Brella SweatControl Patches are a sweat reduction alternative to Botox injections for those who wish for a needle-free treatment.
What is the best over-the-counter treatment for sweating?
Brella SweatControl patches are an in-office treatment option, meaning they will not be available over the counter and must be performed by a trained healthcare provider, such as Dr. Michele Green. Patients looking for an over-the-counter treatment option must turn to topical antiperspirants, gels, sprays, or wipes, such as those containing aluminum chloride hexahydrate. The aluminum in these treatments blocks the sweat glands from secreting sweat mechanically, meaning the blockage is cleared when more sweat is produced, and the medicine must be reapplied. Over-the-counter treatments are most effective for patients with an HDSS score of 1 or 2. Patients with a higher HDSS score often require treatments such as Botox or Brella to reduce sweating effectively.
Patients can also make lifestyle changes that can help to make excessive sweating less stressful in day-to-day life by completing the following adjustments:
- Dress: Wear clothing made of cotton that is loose-fitting
- Environment: Stay cool and away from crowded places
- Food: Avoid spicy foods and alcoholic beverages
- Emotionally: Avoid stressful situations when possible and practice techniques to reduce feelings of stress.
What is the FDA-approved treatment for hyperhidrosis?
Two FDA-approved treatments for excessive underarm sweating and hyperhidrosis are available (or soon available) at Dr. Green’s office: Botox treatment and Brella SweatControl patches. Botox is an injectable treatment composed of the neurotoxin onabotulinum toxin type A, which acts as a neuromodulator when injected into the treatment area. This means that the botulinum toxin in the injection blocks nerve endings at the injection site and prevents the sweat glands from secreting sweat in the case of hyperhidrosis treatment. Botox is highly effective at treating hyperhidrosis, and one randomized placebo-control study demonstrated that 94% of patients who received Botox treatment for underarm sweating saw a 50% or more reduction in sweating. Further, more than 80% were still experiencing a 50% reduction in sweating four months after receiving the treatment. While the treatment effects eventually fade, receiving repeat Botox treatments for hyperhidrosis can help reduce sweating and improve quality of life. Dr. Green is an expert Botox injector with years of experience treating patients with hyperhidrosis.
What is the new treatment for hyperhidrosis?
Created by Candesant Biomedical, Brella SweatControl patches are the newest FDA-approved treatment for excessive underarm sweating. Harnessing the scientific principle that heat is produced when alkali metals mix with water, the sodium sheet is placed on the armpit with the help of an adhesive overlay, and with targeted alkali thermolysis – or TAT technology – the alkali mixes with the water, creating controlled heat damage to the sweat glands. This targeted damage causes a reduction in sweat production by as much as 50% for up to four months without the need for injections or surgery or a risk of developing serious adverse events.
When will Brella be available for patients?
Brella SweatControl Patch, created with the patented targeted alkali thermolysis technology by Candesant Biomedical, has recently been FDA-cleared to treat excessive underarm sweating. The treatment will be made available to certain dermatologists and other healthcare providers in January 2024. As soon as the treatment is available, board-certified dermatologist Dr. Michele Green will have these Brella SweatControl patches available for her NYC dermatology patients. With the 3-minute SweatControl Patch, patients can see up to a 50% reduction in sweat production for up to 4 months. These patches should help significantly reduce hyperhidrosis and can be combined with other treatment modalities.
Dr. Michele Green is an internationally renowned board-certified dermatologist with over 25 years of experience providing her patients with the best non-invasive treatment options, including for hyperhidrosis. Castle Connolly, Super Doctors, and New York Magazine consistently identify Dr. Green as one of NYC’s best dermatologists for her dedication to her patients and expertise. When you consult with Dr. Green, she will work with you to create a completely customized treatment plan that combines the in-office procedures and specially formulated skincare products best suited to your specific needs and goals. To find out more about Candesant Biomedical’s new product, visit candesant.com. To learn more about treating hyperhidrosis, Brella, and other cosmetic non-invasive procedures, call 212 535 3088 or contact us online to book an appointment today.
 212-535-3088
212-535-3088